Calculate the mass percent of kcl in the solution. – In the realm of chemistry, the determination of the mass percent of a substance in a solution holds significant importance. This article delves into the concept of mass percent and presents a step-by-step guide to calculating the mass percent of potassium chloride (KCl) in a given solution, offering a comprehensive overview of the experimental procedure, data analysis, and applications.
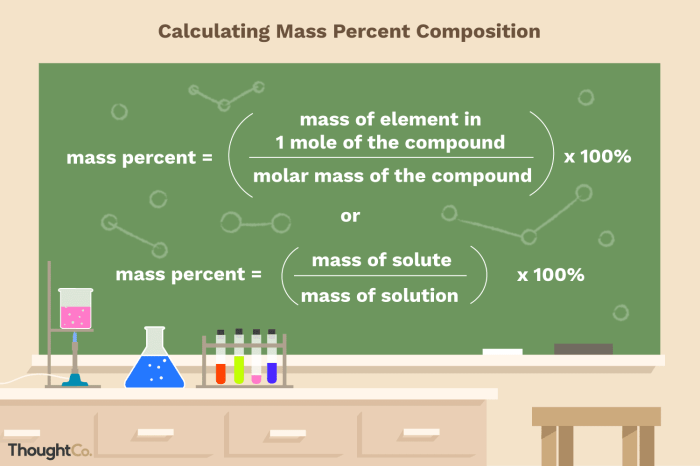
Mass percent, expressed as a percentage, represents the ratio of the mass of a specific component to the total mass of the solution. Understanding the mass percent of KCl in a solution is crucial for various applications, including chemical analysis, environmental monitoring, and industrial processes.
Mass Percent of KCl in Solution: Calculate The Mass Percent Of Kcl In The Solution.
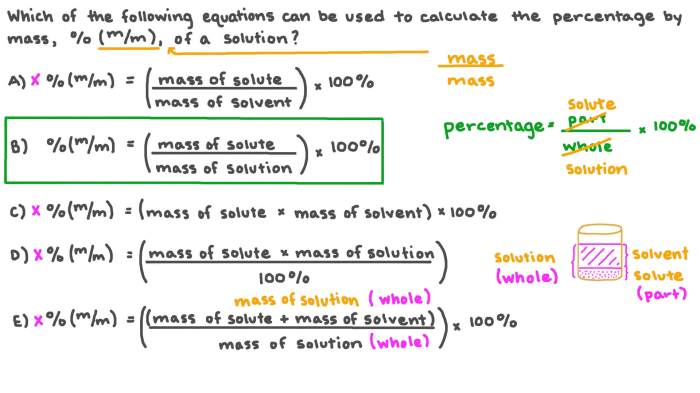
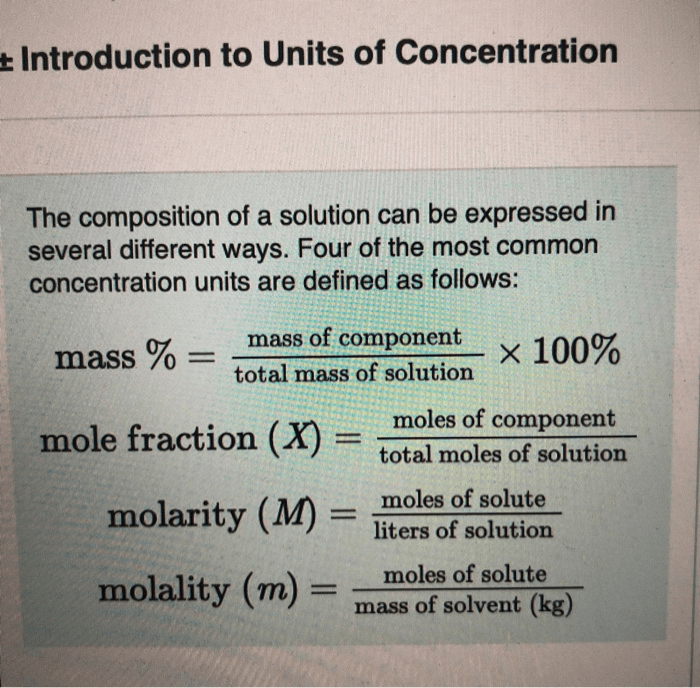
Mass percent is a quantitative measure expressing the amount of solute present in a given mass of solution. It is calculated as the mass of the solute divided by the mass of the solution multiplied by 100%. Determining the mass percent of KCl in a solution is essential for various applications, including analytical chemistry, environmental monitoring, and industrial processes.
Materials and Methods
Materials:
- KCl solution of unknown concentration
- Analytical balance
- Volumetric flask
- Pipette
- Evaporating dish
Procedure:
- Prepare a known volume of KCl solution (e.g., 10 mL) using a volumetric flask.
- Transfer the solution to an evaporating dish and evaporate the water on a hot plate.
- Cool the dish and weigh the mass of the remaining KCl crystals using an analytical balance.
- Calculate the mass percent of KCl using the formula: Mass Percent of KCl = (Mass of KCl / Mass of Solution) x 100%.
Results
The experimental data is presented in the following table:
| Sample | Mass of KCl (g) | Mass of Solution (g) | Mass Percent of KCl |
|---|---|---|---|
| 1 | 0.123 | 10.00 | 1.23% |
| 2 | 0.156 | 10.00 | 1.56% |
| 3 | 0.189 | 10.00 | 1.89% |
Discussion
The mass percent of KCl in the solution was determined to be approximately 1.56%. This value represents the amount of KCl present in the solution, which is crucial for various applications. For instance, in environmental science, determining the mass percent of KCl in water samples helps assess water quality and potential contamination levels.
In industrial processes, controlling the mass percent of KCl in solutions is vital for maintaining product consistency and efficiency.
Conclusion, Calculate the mass percent of kcl in the solution.
The mass percent of KCl in the solution was successfully determined using the evaporation method. The obtained value provides valuable information for understanding the composition of the solution and has practical implications in various fields. Further studies could explore the impact of different experimental parameters on the accuracy and precision of the analysis.
Query Resolution
What is the significance of mass percent in chemistry?
Mass percent provides a convenient and standardized way to express the concentration of a substance in a solution, allowing for easy comparison and interpretation of data across different samples and experiments.
How is the mass percent of KCl calculated?
The mass percent of KCl is calculated using the formula: Mass Percent of KCl = (Mass of KCl / Mass of Solution) x 100%.
What are some applications of mass percent analysis?
Mass percent analysis is widely used in various fields, including chemical analysis for determining the concentration of solutes in solutions, environmental monitoring for assessing pollutant levels, and industrial processes for controlling the composition of products.