Of the reactions below which one is a decomposition reaction – Of the reactions below, which one is a decomposition reaction? This question delves into the fascinating world of chemical reactions, where substances undergo transformations, forming new compounds or breaking down into simpler ones. Decomposition reactions, a specific type of chemical reaction, involve the breakdown of a compound into simpler substances.
Join us as we explore the intricacies of decomposition reactions, unraveling their types, factors influencing them, and their practical applications in various industries.
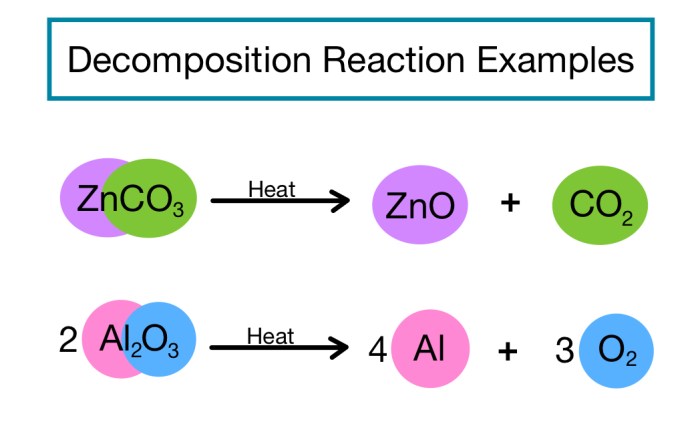
Decomposition Reactions
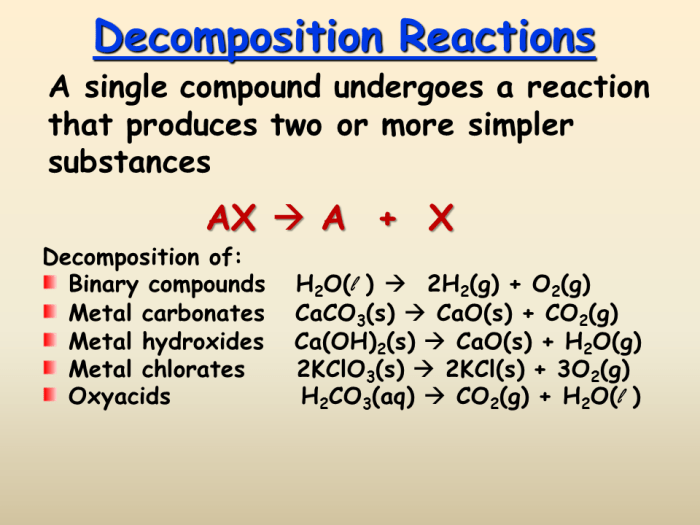
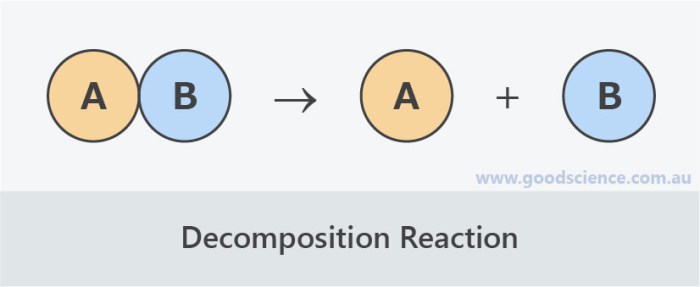
Decomposition reactions are chemical reactions in which a single compound breaks down into two or more simpler compounds. This process is often accompanied by the absorption of energy.
A general equation for a decomposition reaction is:
AB → A + B
where AB is the reactant compound and A and B are the products.
Types of Decomposition Reactions, Of the reactions below which one is a decomposition reaction
There are three main types of decomposition reactions:
- Thermal decompositionoccurs when a compound decomposes due to the application of heat.
- Photodecompositionoccurs when a compound decomposes due to the absorption of light energy.
- Electrolytic decompositionoccurs when a compound decomposes due to the passage of an electric current.
Factors Affecting Decomposition Reactions
The rate of a decomposition reaction can be affected by several factors, including:
- Temperature: Higher temperatures generally increase the rate of decomposition.
- Concentration: Higher concentrations of the reactant compound increase the rate of decomposition.
- Surface area: Increasing the surface area of the reactant compound increases the rate of decomposition.
- Catalysts: Catalysts are substances that increase the rate of a reaction without being consumed themselves. They can be used to accelerate decomposition reactions.
Applications of Decomposition Reactions
Decomposition reactions have a wide range of applications, including:
- Extraction of metalsfrom their ores
- Production of gasessuch as hydrogen and oxygen
- Manufacture of chemicalssuch as ammonia and sulfuric acid
- Decomposition of organic matterin composting and digestion
Example Reactions
| Reaction | Type of Decomposition Reaction | Description |
|---|---|---|
| 2H2O → 2H2 + O2 | Thermal decomposition | Water decomposes into hydrogen and oxygen when heated. |
| 2AgCl → 2Ag + Cl2 | Photodecomposition | Silver chloride decomposes into silver and chlorine when exposed to light. |
| 2NaCl → 2Na + Cl2 | Electrolytic decomposition | Sodium chloride decomposes into sodium and chlorine when an electric current is passed through it. |
Answers to Common Questions: Of The Reactions Below Which One Is A Decomposition Reaction
What are the key characteristics of decomposition reactions?
Decomposition reactions involve the breakdown of a compound into simpler substances, often accompanied by the release of energy.
How do different factors influence the rate of decomposition reactions?
Factors such as temperature, surface area, concentration, and the presence of a catalyst can significantly affect the rate of decomposition reactions.
Can you provide an example of a decomposition reaction used in everyday life?
The decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2) is a common example used in household cleaning products.